| Description
|
Livestock Breeding and Veterinary Department
Ministry of Agriculture, Livestock and Irrigation
Procedure for the Importation of Animal Semen into Myanmar
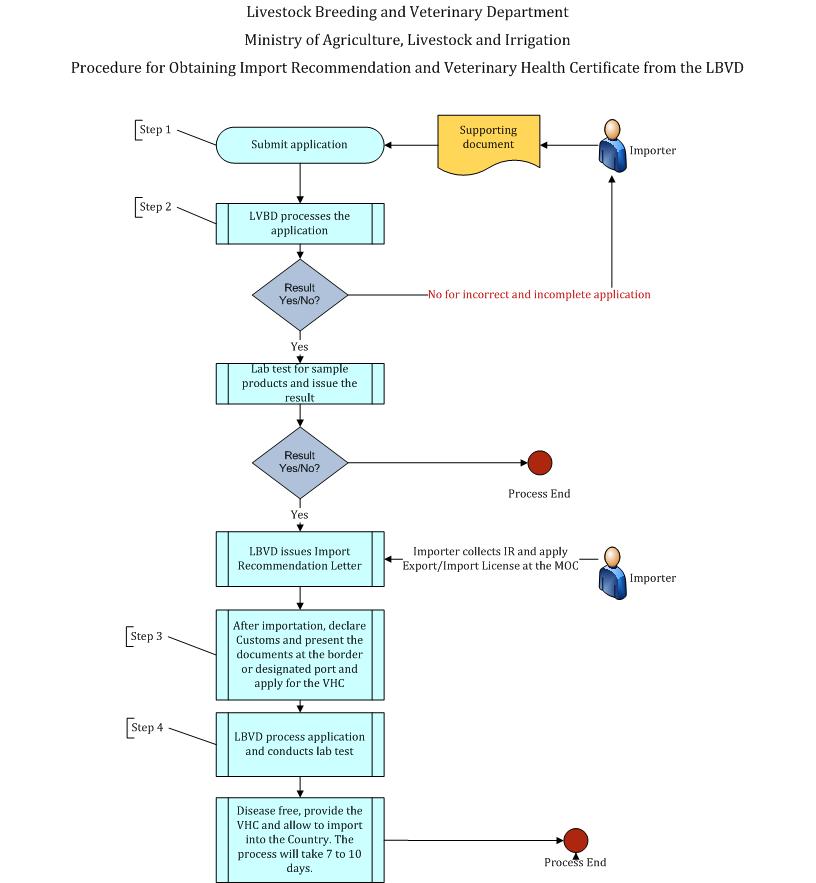
Step (1)
A person importing Animal Semen shall apply a recommendation certificate to the Livestock Breeding and Veterinary Department (LBVD) along with the following supporting documents.
- Application letter with Company letterhead signed by the company’s director
- Copy of Veterinary Health Certificate issued by the Veterinary authority of exporting country
- Farm registration certificate (if applicable)
- Performa Invoice
- Sale Contract
- Recommendation from the Myanmar Livestock Federation
- Copy of company registration issued by Directorate of Investment and Company Administration
- Form VI
- Form XXVI
- Certificate containing a full description or identification of the animals
Step (2)
LBVD shall check and review on the completeness of the application and register the documents. For the incomplete and incorrect application, LBVD informs the importer with reason. The importer corrects, completes and resubmit the documents. If application is rejected, the process ends here. For the complete and correct application, the LBVD shall issue the import recommendation certificate. After receiving the import recommendation certificate from the LBVD, the importer shall continue to apply the import license at the Ministry of Commerce.
Step (3)
Upon the arrival to designated port or border gate of Myanmar, the importer shall submit the Import Declaration and relevant documents to the Customs Department and the LBVD for laboratory test through Myanmar Automated Cargo Clearance System (MACCS). LBVD shall test the animal semen and check the health certificate signed by authorized Veterinary Officer, including the individual identification of importing animal semen, recommendation certificate issued by the LBVD, import license issued by the Department of Trade, MOC, original Veterinary Health Certificate issued by the Veterinary Authority of exporting country, packing list and bill of lading/Airway bill and PC3 for border trade importation.
Step (4)
If the lab test is proved with the negative result from the described diseases, LBVD shall issue the Veterinary Health Certificate (VHC) to the importer. LBVD will provide the result in the MACCS system. The LBVD shall, with the approval of the Ministry establish Inspection Stations in required regions for inspection of imported live animals. The importer shall pay the quarantine fee, laboratory testing fee and Health Certificate fee (2 % of the value of straw cost) as determined by the LBVD.
Remark-
The overall process will take from 7 to 10 days.
- A health certificate signed by a full (time) authorized veterinary official of the government of the exporting country or endorsed by the said veterinary in case the certificate was previously acknowledge by a veterinary supervisor of the semen production center stating;
- Name and address of the semen production center and premises of origin
- Number of species and breeds
- Identify of the donor animal
- Dates of semen collection
- A certificate should contain;
- The country of origin has been free from African Swine Fever and Rinderpest during the part 3 (three) years prior to the collection of semen for export to Myanmar and until date of dispatch of the semen to Myanmar.
- The country/ origin/ zone of origin has been free from Foot and Mouth Disease (FMD) and officially approved by the Office International des Epizooties (OIE).
- The semen must be produced from semen production center licensed by the veterinary authority of the government of the exporting country and must be under the direct supervision and sanitary control of authorized veterinary official.
- Male animal and teaser animal shall only enter the semen production center if they are found to be free from clinical signs or other evidences of Tuberculosis, Brucellosis, Leptospirosis, Vesicular Exanthema, Vesicular Stomatitis, Swine Vesicular Influenza, Hog Cholera (Classical Swine Fever), Aujeszky’s Disease, Porcine Parvovirus Infection, Porcine Reproductive and Respiratory Syndrome (PRRS), Atrophic Rhinitis, Transmissible Gasteroenteritis and Teschen Disease during the (twelve) months prior to the collection until the date of dispath of the semen to Myanmar.
- The animals must be clinically healthy and physiologically normal and must pass pre-entry test within the 30 (thirty) days prior to entry into isolation at the semen production center of the following diseases Bovine Tuberculosis and Brucellosis (Brucella aborts, Brucella susis).
- The donor male animals and any teaser animals used in the collection of the semen must remain in isolation at the AI center for a period of at least 30 (thirty) days before being retested for the following disease with negative results using method or other methods;
- Tuberculosis: the animals were kept in a country part of the territory of a country or AI center where all animals are officially free from bovine tubercullosis and when the routine tests are being applied at least every 12 (twelve) months.
- Brucellosis: the animals were kept in a country, part of the territory of the country or AI center where all animals are officially free Brucellosis (Brucella abortus, Brucella susis) and which the routine tests are being applied at least every 12 (twelve) months.
- Aujeszky’s Diseases
- Transmissible Gastroenteritis
- Teschen Disease
- Swine Vesicular Disease
- PRRS
- Leptospirosis
- The semen must be obtained from the donor animals with normal libido and which have the records of their fertility as well as the records of their progeny possible to determine that it is no associated with any genetic defect.
- Preservatives and antibiotics including any mycoplasmacidal drugs used in the diluents must be clearly declared.
- The semen was collected, processed and stored strictly in accordance with the relevant criteria set out in the OIE International Animal Health code.
- Failure to follow the import procedures may result in returning the semen to the country of origin or destroying without compensation.
|