Food Import Recommendation
| Category | Import |
| Picture |
|
| Summary | Obtaining Food Import Recommendation from Food and Drug Administration under the Ministry of Health |
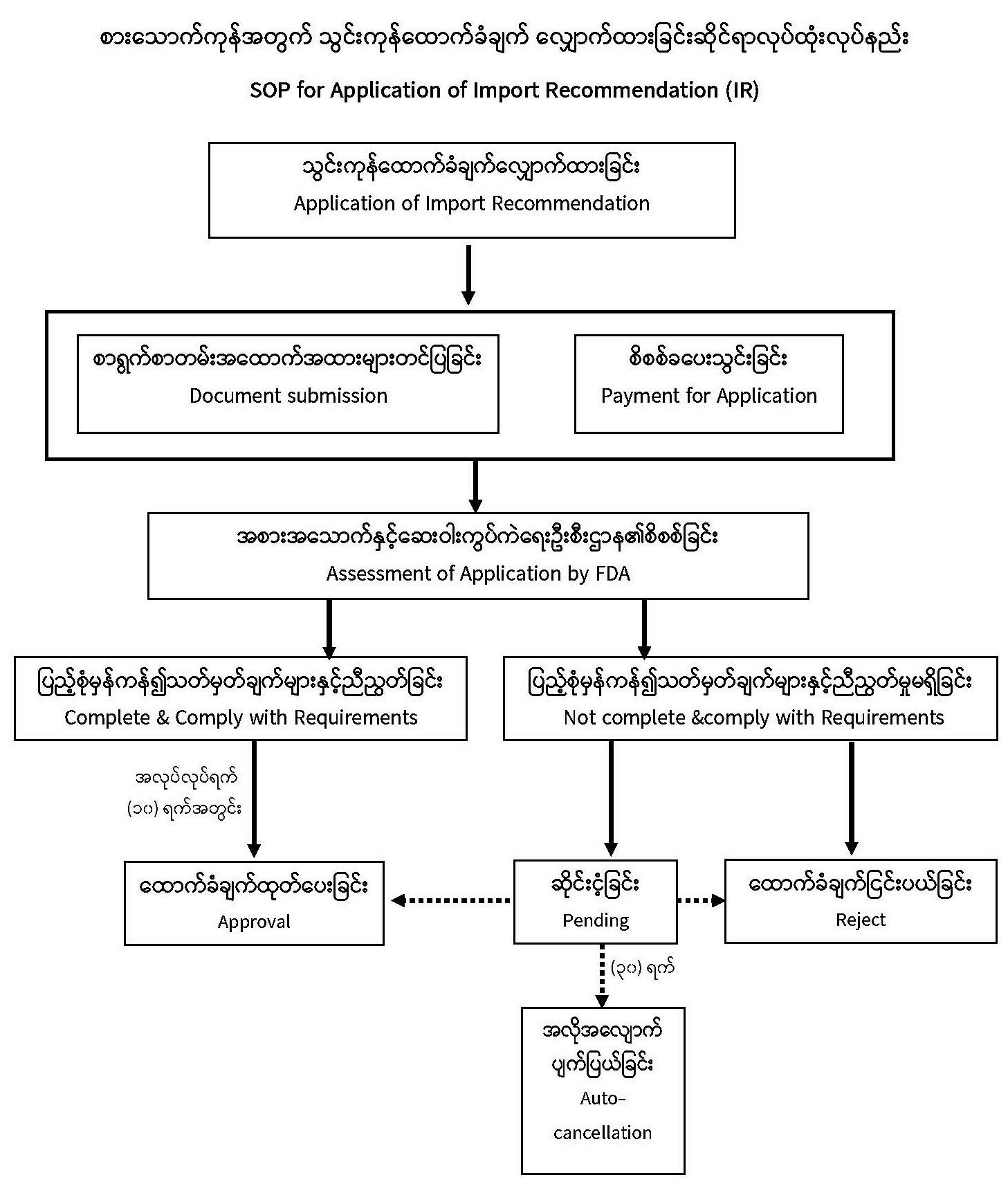
| Description | Food and Drug Administration Ministry of Health Procedures for Obtaining Food Import Recommendation (IR)
Importers need to obtain, complete and submit application for Import Recommendation (IR) along with necessary supporting documents to the Department of Food and Drug Administration (FDA) under the Ministry of Health. Application of individual IR is required for every single type of food item and the applicant user guide for Import Recommendation is provided here |
Related Documents
| File Name | Language | File Size | Download |
|---|
Forms
The following form/s are used in this procedure.
| Title | Description | Category | Agency |
|---|---|---|---|
| Application Form for obtaining Export Recommendation from MOHA for the exportation of Controlled Chemical Substances (Form 7) | Anyone who wants to export controlled chemical substances shall obtain the export recommendation from the Supervisory Committee for Controlled Precursor by submitting this application form, Form 7. | Export | Ministry of Home Affairs |
| Application format for Import Recommendation to Department of Food and Drug Administration (FDA) | The importers have to apply import recommendation for food importation to Department of Food and Drug Administration (FDA) by using the sample as per attached. | Import | Department of Food and Drug Administration |
| Application format for Import Health Recommendation to Department of Food and Drug Administration(FDA) | The importers have to apply health recommendation for importation of Foodstuffs to the Department of Food and Drug Administration (FDA) by using the sample format as per attached. | Import | Department of Food and Drug Administration |
| Undertaking Letter | In order to guarantee the quality of food imported, the importers have to submit undertaking letter to the Department of Food and Drug Administration (FDA) as per attached format. | Import | Department of Food and Drug Administration |
